Nitrogen Dioxide Gas Sterilization Market Outlook 2026–2036 Driven by Low-Temperature Device Processing
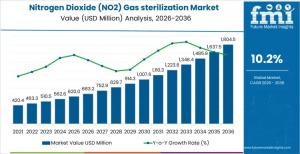
Global NO₂ gas sterilization market projected to grow from USD 683.2 million in 2026 to USD 1.8 billion by 2036 at 10.2% CAGR.
NEWARK, DE, UNITED STATES, January 16, 2026 /EINPresswire.com/ -- The global nitrogen dioxide (NO₂) gas sterilization market is projected to reach USD 683.2 million in 2026 and expand to USD 1,804.5 million by 2036, growing at a compound annual growth rate (CAGR) of 10.2%. The market’s growth reflects increasing reliance on low-temperature sterilization technologies designed for heat- and moisture-sensitive medical devices, electronics, and polymer-based assemblies. Adoption remains focused on regulated healthcare and manufacturing environments where validated microbial inactivation, short cycle times, and material compatibility are operational priorities.
NO₂ gas sterilization is used primarily in medical device manufacturing, hospital central sterile services departments (CSSDs), and contract sterilization facilities. Value generation is closely tied to the placement of capital equipment, supported by recurring demand for sterilant consumables, monitoring systems, validation services, and maintenance contracts aligned with regulatory compliance and quality system requirements.
Explore comprehensive market trends, regional outlooks, and competitive intelligence Get a Sample Copy Now - https://www.futuremarketinsights.com/reports/sample/rep-gb-31411
Market Context: Why NO₂ Gas Sterilization Is Gaining Attention
Demand for nitrogen dioxide gas sterilization is rising as healthcare providers, pharmaceutical manufacturers, and medical device producers seek effective sterilization methods that avoid the thermal stress and moisture exposure associated with steam processes. Compared with ethylene oxide, NO₂ offers shorter cycle times and reduced concerns around prolonged aeration and residue management for specific applications.
Facilities managing complex instruments, electronics, and polymer-based devices increasingly specify NO₂ systems that deliver validated sterility assurance levels while preserving device integrity. Procurement decisions emphasize cycle reproducibility, integration with cleanroom operations, environmental monitoring, and predictable performance under regulated conditions. These factors are particularly relevant in settings handling high instrument throughput and requiring rapid turnaround supported by documented sterilization histories.
Growth in minimally invasive procedures and the expansion of outpatient surgical centers are reinforcing the role of NO₂ gas sterilization within diversified sterilization portfolios rather than as a universal replacement for established technologies.
Market Size and Key Statistics
• Market value (2026): USD 683.2 million
• Forecast value (2036): USD 1,804.5 million
• CAGR (2026–2036): 10.2%
• Leading product by demand share: NO₂ sterilization chambers
• Fastest-growing countries: India, Brazil, China, United States, Germany
Product Segmentation Highlights
NO₂ sterilization chambers account for approximately 26.0% of total demand, reflecting their role as the core infrastructure for controlled sterilization cycles. Sterilant consumables represent 22.0% of the market, driven by recurring usage linked to cycle frequency and throughput. Indicators and monitoring systems, along with maintenance and service contracts, each account for 18.0%, underscoring the importance of compliance verification and uptime assurance. Validation and compliance services comprise the remaining 16.0%, supporting regulatory alignment across healthcare and manufacturing settings.
Application and End-User Trends
Low-temperature medical device sterilization represents the largest application segment at 34.0%, driven by demand for processing polymer-based and electronic devices. Hospital point-of-use sterilization accounts for 22.0%, supporting faster clinical turnaround. Medical device manufacturing contributes 20.0%, while contract sterilization services represent 14.0%, serving multiple clients through centralized capacity.
Hospitals and CSSD units account for 28.0% of end-user demand, followed closely by medical device manufacturers at 26.0% and contract sterilization providers at 22.0%. These segments reflect where operational control, regulatory accountability, and throughput requirements intersect most strongly.
Regional Growth Outlook
Global expansion remains selective and application-driven. India leads growth with a projected CAGR of 12.7%, supported by domestic medical device manufacturing expansion and export compliance needs. Brazil follows at 12.2%, driven by growth in single-use devices and regional sterilization services. China is forecast to grow at 11.7%, aligned with large-scale, export-oriented device manufacturing. The United States and Germany show steady growth at 8.8% and 8.6%, respectively, reflecting targeted adoption for material-sensitive devices and compliance-focused workflows.
Competitive Landscape
The competitive environment includes established sterilization technology providers such as Noxilizer, STERIS, Getinge, Advanced Sterilization Products, and Fedegari. Competitive differentiation centers on cycle efficiency, material compatibility, regulatory alignment, and the ability to support validation, monitoring, and compliance requirements. Buyer evaluations focus on microbial lethality, installation complexity, footprint constraints, and suitability for lumened and polymer-based devices.
Complete Market Report Ready for Access : https://www.futuremarketinsights.com/checkout/31411
Outlook
The nitrogen dioxide gas sterilization market is expected to maintain sustained growth through 2036, driven by material compatibility needs, throughput efficiency, and regulatory scrutiny of alternative sterilization modalities. Adoption will continue to depend on regulatory acceptance, infrastructure readiness, and economic justification, reinforcing NO₂’s role as a specialized, application-focused solution within the broader sterilization landscape.
Why FMI: https://www.futuremarketinsights.com/why-fmi
Related Insights from Future Market Insights (FMI)
United Kingdom 3D Bioprinted Human Tissue Market: https://www.futuremarketinsights.com/reports/united-kingdom-3d-bioprinted-human-tissue-market
Instrument Cleaning Chemistries Market: https://www.futuremarketinsights.com/reports/instrument-cleaning-chemistries-market
Australia and New Zealand Cold Laser Therapy Market: https://www.futuremarketinsights.com/reports/australia-and-new-zealand-cold-laser-therapy-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.